Abstract
Background: Myeloma patients (pts) with renal insufficiency (RI) have historically inferior disease outcomes; up to 50% have acute kidney injury (AKI) at diagnosis. AKI is a direct insult from light chains causing cast nephropathy; and physicochemical light chain properties such as self-association and aggregate formation may determine severity of injury. Prior induction treatment data in the AKI myeloma population does not reflect newer agents and mechanisms of action with improved activity and favorable clinical pharmacology profiles for renal use. Daratumumab has broad applications based upon extensive data in myeloma, reduces circulating light chains, is associated with preserved renal function in AL amyloidosis and is cleared by non-renal routes. Bortezomib, dexamethasone, and lenalidomide are effective induction antimyeloma agents in common use. We hypothesized early therapy with full doses of daratumumab and these 3 agents would improve myeloma-induced AKI in a population rarely included in clinical trials.
Methods: We initiated a Simon's two-stage design trial in newly diagnosed pts with severe AKI (CrCl < 30 mL/min) using 4 x 21-day cycles of induction with daratumumab 16 mg/kg IV weekly x 3 (C1-3, C4D1 only), bortezomib 1.3 mg/m2 SQ days 1, 4, 8, and 11, and dexamethasone 40 mg (reduced to 20 mg at C2 for > 75 yrs.) days 1-4 (C1 only, D1 only C2-4), 8, and 15, with add-on lenalidomide at C2 (25 mg if CrCl > 30 mL/min) due to logistics of obtaining supply for inpatients. Standard antiviral, antithrombotic, and premedication regimens were used. Eligibility criteria were broad [CrCl < 30 mL/min, PS 0-2, ANC > 1000/mm3, Hgb > 7 g/dL (transfusion allowed > 7 days prior), plt > 75,000/mm3 transfusion allowed > 3 days prior) and dose reduction strategies liberal for generalizability. Primary objective was to determine the proportion with renal function recovery (CrCl > 50 mL/min) after 2 cycles; secondary objectives were best response by IMWG criteria, renal function at end of treatment, dose density/tolerability, adverse event (AE) frequency and severity, renal function changes by multiple estimates [Cockcroft-Gault (C-G), MDRD, 24-hour urine, and CKD-EPI], and daratumumab pharmacokinetics. If > 7 responses were seen in the first 11 pts, an additional 14 would be accrued. These data reflect the planned analysis of the initial cohort.
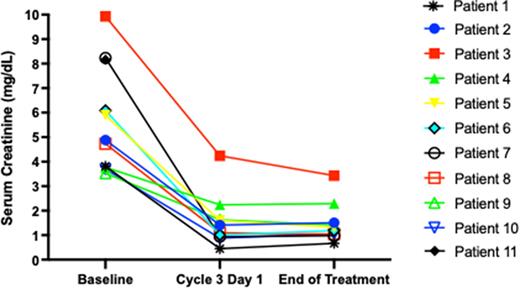
Results: Thirteen pts have been enrolled: 8 male, 7 white/6 black, median age 69 yr (46-82), 11 treated and evaluable for the primary endpoint. Median baseline CrCl was 13.8 mL/min (range 4.9-20.2), median serum creatinine (SCr) 4.92 mg/dL (range 3.53-9.93). One patient withdrew consent, and 1 was inevaluable due to rapid disease progression and death. Seven achieved CrCl improvement to > 50 mL/min (median C3D1 CrCl 61 mL/min, range 22-151) after 2 cycles, all had improvement in SCr at C3D1 (Figure). Lenalidomide dose was 25 mg on C2D1 in 9/11 pts. Median (range) dose density (delivered/planned) for each agent was daratumumab 100% (40-100), bortezomib 100% (25-100), dexamethasone 80% (67-100), and lenalidomide 100% (0-100). The overall response rate by IMWG criteria was 100%, with a > VGPR rate of 82%. Best responses were CR (3), VGPR (6), PR (2). Treatment-emergent grade ¾ hematologic AEs were anemia (90.9%), lymphopenia (81.8%), thrombocytopenia (36.4%), and neutropenia (9.1%). All grade treatment-emergent non-hematologic AEs were fatigue (n=4), infusion reaction (n=3), hyponatremia (n=2), rash, transaminitis, peripheral neuropathy, hyperglycemia, weakness, hypokalemia, hypophosphatemia, dyspnea, fever, edema (n=1 each).
Conclusions: Early, aggressive therapy with a daratumumab-based induction regimen in myeloma pts with severe AKI improves renal function, with the majority achieving CrCl > 50 mL/min after 2 cycles. In addition, patients achieved an ORR of 100%, with most patients achieving CR or VGPR. Adverse events seen were consistent with prior experience with the 4-drug regimen. Per protocol, 14 additional patients will be accrued in the second stage of the study.
Disclosures
Joseph:BMS: Research Funding; GSK: Research Funding; Janssen: Research Funding. Kaufman:AbbVie: Other: Member of steering committee; AbbVie, Genentech, and Bristol Myers Squibb: Consultancy; Incyte: Other: Member of data safety monitoring committee . Hofmeister:Janssen: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; BlueBird Bio: Honoraria; Genzyme: Membership on an entity's Board of Directors or advisory committees. Dhodapkar:Lava Therapeutics, Sanofi, Janssen: Membership on an entity's Board of Directors or advisory committees. Lonial:Novartis, BMS, GSK, Amgen, Merck, Janssen: Honoraria; Celgene, Janssen, Takeda: Research Funding; AbbVie, Bluebird, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis Pharma, and Takeda.: Consultancy. Nooka:Bristol-Myers Squibb, Janssen, Takeda, Amgen, Adaptive, GlaxoSmithKline, Sanofi, Oncopeptides, Karyophram, SecureBio, and BeyondSprings: Consultancy, Honoraria.
OffLabel Disclosure:
Daratumumab with bortezomib, lenalidomide, and dexamethasone in newly diagnosed patients with CrCL < 30 mL/min
Author notes
Asterisk with author names denotes non-ASH members.